240 volunteers injected in Vietnam vaccine phase 3 trial
(VOVWORLD) - The Military Medical Academy on Friday continued to test the home-grown Nanocovax vaccine against COVID-19 in its phase 3 trial for the first 240 volunteers.
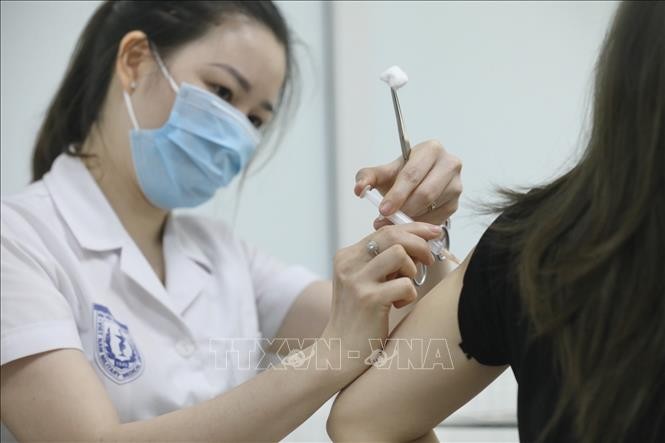 A medical staff injects Nanocovax vaccine against COVID-19 to volunteers participating in the 3rd phase trial at the Military Medical Academy. Photo: VNA A medical staff injects Nanocovax vaccine against COVID-19 to volunteers participating in the 3rd phase trial at the Military Medical Academy. Photo: VNA
|
Nanocovax is Vietnam's first Covid-19 vaccine officially entering phase 3 human trial, which will test the vaccine for about 13,000 volunteers.
Nanocovax developed by the Ho Chi Minh City-based Nanogen Company since mid-December 2020 is Vietnam's first COVID-19 vaccine to be tested into human clinical trials.
More than 6,000 volunteers have registered to participate in the trial at the Military Medical Academy, of which more than 600 will be screened to be eligible to get the shots. Initially, the Academy will test the Nanocovax vaccine for 1,000 volunteers, divided into 2 groups, one group will be injected a dose of 25mcg and the other group to be injected a placebo to compare the protective efficacy of the vaccine.