Pfizer, BioNTech seek US COVID-19 vaccine clearance for children 5-11
(VOVWORLD) - Pfizer and BioNTech have asked US regulators to authorize emergency use of their COVID-19 vaccine for children ages 5 to 11, Pfizer said on Thursday.
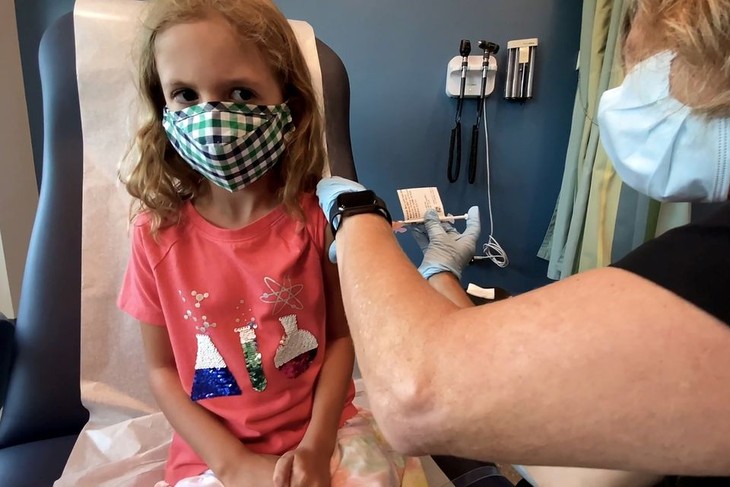 Lydia Melo, 7, is inoculated with one of two reduced 10 ug doses of the Pfizer BioNtech COVID-19 vaccine during a trial at Duke University in Durham, North Carolina, September 28, 2021 in a still image from video. (Photo: REUTERS) Lydia Melo, 7, is inoculated with one of two reduced 10 ug doses of the Pfizer BioNtech COVID-19 vaccine during a trial at Duke University in Durham, North Carolina, September 28, 2021 in a still image from video. (Photo: REUTERS)
|
The US Food and Drug Administration has set a date of Oct. 26 for its panel of outside advisers to discuss the application, making it possible for children in this age group to begin receiving the two-dose Pfizer/BioNTech vaccine shortly afterward.
Pfizer wrote on Twitter that "with new cases in children in the US continuing to be at a high level, this submission is an important step in its ongoing effort against COVID19."
The vaccine has already won US emergency use authorization in teens ages 12 to 15 and is fully approved by regulators for people ages 16 and up.
The vaccine could be ready for roll out next month pending approval from federal health agencies.